n3- protons neutrons electrons|How to find Protons & Electrons for the Nitride ion (N 3 : Pilipinas The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The .
Autodesk Maya is professional 3D software for creating realistic characters and blockbuster-worthy effects. Bring believable characters to life with engaging animation tools. Shape 3D objects and scenes with intuitive modeling tools. Create realistic effects—from explosions to cloth simulation.
PH0 · Protons, neutrons, and electrons in atoms (video)
PH1 · Number of Protons, Neutrons, and Electrons in an Atom
PH2 · Number of Protons, Neutrons, and Electrons in an Atom
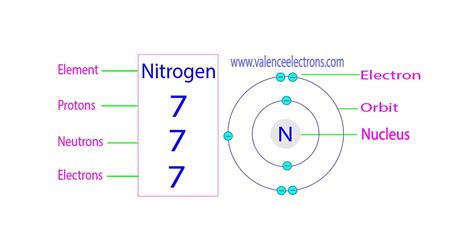
PH3 · Nitrogen
PH4 · How to find the number of protons, neutron, and electrons.
PH5 · How to find Protons & Electrons for the Nitride ion (N 3
PH6 · How to Find the Number of Protons, Neutrons, and Electrons
PH7 · How to Find the Number of Protons, Neutrons, and
PH8 · How To Calculate The Number of Protons, Neutrons, and Electrons
PH9 · How To Calculate The Number of Protons, Neutrons,
PH10 · Determine the number of protons (p) and electrons (e) in N3−
PH11 · 4.4: Protons, Neutrons, and Electrons
PH12 · 3.3: Subatomic Particles
PH13 · 2.6: Protons, Neutrons, and Electrons in Atoms
https://www.youtube.com/watch?v=q4BK5BunLjQ [Intro] G Gsus4 G Gsus4 [Verse 1] G I love you, Lord C G For your mercy never fails me D/F# Em C D All my days, I’ve .
n3- protons neutrons electrons*******In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the Nitride ion (N3-). From the Periodic Ta.Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Electrons are found outside the nucleus. Protons are positively charged and have a .
Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and .The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons .Learning Objectives. Know the basics of the experiments involving the discoveries of the three subatomic particles. Memorize relative charge values and amu masses of the .How to find Protons & Electrons for the Nitride ion (N 3 The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The .
Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron). The .Textbook Question. Some ions do not have a corresponding neutral atom that has the same electron configuration. For each of the following ions, identify the neutral atom that has .From the periodic table, we find that it has 29 protons. The mass number (65) is the sum of the number of protons and neutrons. Therefore, we can subtract the number of protons from the atomic number to get the .Question: 15 N3- Indicate the number of protons, neutrons, and electrons in the following atom: O 7 protons, 8 neutrons, 10 electrons O 7 protons, 8 neutrons, 4 electrons O 15 protons, 3 neutrons, 15 electrons O 8 protons, 7 neutrons, 11 electrons. Show transcribed image text. Here’s the best way to solve it. Expert-verified.
Watch this video to learn how protons, neutrons, and electrons are arranged in atoms, and how the number and distribution of these subatomic particles determine the identity and properties of .Figure 3.3.2 3.3. 2: Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Masses for the three subatomic particles can be expressed in amu ( atomic mass units) or grams. For simplicity, we will use the amu unit for .We would like to show you a description here but the site won’t allow us.A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter.It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg (938.272 MeV/c 2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.Table 1.15.1 1.15. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can see, the mass . Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can .Study with Quizlet and memorize flashcards containing terms like Specify the number of protons, neutrons, and electrons in the neutral atom bromine-80., The ion N3− has _____ protons and _____ electrons., What isotope has 17 protons and 18 neutrons? and more.First, to find the number of protons, we need to realize that the neutral atom had 53 electrons because it is the additional one electron that makes it a 1- anion. Now, because the atom has 53 electrons, it must also have 53 protons, and to find the number of neutrons we subtract this from the mass number. # n = A – # p = 127 – 53 = 74 . Nickel has 28 protons, 31 neutrons and 28 electrons: 29: Copper has 29 protons, 35 neutrons and 29 electrons: 30: Zinc has 30 protons, 35 neutrons and 30 electrons: 31: Gallium has 31 protons, 39 neutrons and 31 electrons: 32: Germanium has 32 protons, 41 neutrons and 32 electrons: 33: Arsenic has 33 protons, 42 neutrons .
Atom is made up of three components namely protons, neutrons, and electrons. Using these components, the identity of the element can be known. The total number of protons and neutrons reveal an element's mass number while the number of protons can reveal its atomic number. . How many protons and electrons are in the ion N3-? Give the .

A atom M have 82 protons, 126 neutrons and 82 electrons, another species N contain 82 protons, 126 neutrons and 80 electrons, then N is a : Medium. View solution > Calculate the total number of electrons present in 1. 4 . Neptunium is the 93rd element of the periodic table so its atomic number is 93. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a .Q. for chlorine, Z = 17,A = 35. give the number of protons, electrons and neutrons in chlorine and chloride ion. Q. the atomic number of Al is 13 and that of Cl is 17. how many electrons, protons, neutrons, are present in Al 3+ and Cl - ions. Q. Find the number of electrons, protons and neutrons present in Mg 12 24. Q. Explain the formation of :-. Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can .
n3- protons neutrons electronsA proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter.It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg (938.272 MeV/c 2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.A nitride ion has 7 protons, 8 neutrons, and 10 electrons. What is the overall charge on this ion? Q. A charged body has 7 electrons and 10 protons. It can be made neutral by adding: . How many protons and electrons are present in C a 2 + ion ? Q. Compare the radii of two species X and Y. (a) X has 12 protons and 12 electrons. (b) Y has 12 .
Frost Mage has traditionally been the DPS spec of choice for leveling, PvP and in early raids due to Fire immunities, falling off in later tiers once Fire Mage can finally deal damage.. Mages in general are full of tricks, such as Polymorph and Blink, and Frost's heavy usage of long slows through Permafrost are what makes Frost the best PvP, .
n3- protons neutrons electrons|How to find Protons & Electrons for the Nitride ion (N 3